Advances and Challenges of the Brazilian Nutrition Labelling Regulation
(ver artículo en español aquí)
Mariana Ribeiro – Instituto Brasileño de Defensa al Consumidor (Idec), Laís Amaral- Instituto Brasileño de Defensa al Consumidor (Idec), Ana Paula Bortoletto – Faculty of Public Health, University of São Paulo
Brazil started discussing improvements to the nutrition food labeling regulation in 2014. The National Health Surveillance Agency (Anvisa, in Portuguese), an independent arm of the Ministry of Health, is responsible for regulating food labeling in the country and led the regulatory process. In all stages of the process, the food and beverage industry tried to influence Anvisa’s decision, through weakening technical discussions and trying to delay the approval of the regulation.
After six years of regulatory process, including a combination of strong and articulated efforts from civil society, the nutrition food labeling regulation was approved: RDC nº 429/2020 and IN nº 75/2020. The process was concluded in October 2020, during a neoliberal government and the COVID-19 pandemic, but only started to take effect two years later in October 2022. Considering its specifications (items already on the market; new products; homemade products, beverages in returnable packaging), the products have different deadlines resulting in five years for all the companies to adapt their labels (until October 2025).
The new regulation excludes unprocessed and minimally processed foods, which is aligned to the Brazilian Dietary Guidelines, and the main changes comprise:
1) FoPNL: Mandatory FoPNL in the format of a magnifying glass, positioned on the top half of the main panel of the food package, in black drawing on a white background, to highlight products that are “high in” added sugar, saturated fat and sodium.
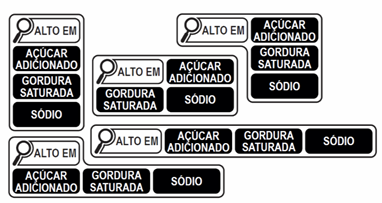
Figure 1: Anvisa´s final position of FOPNL design. (2021)

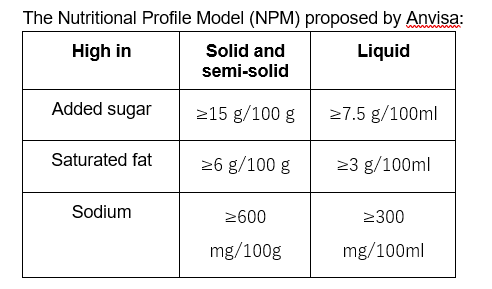
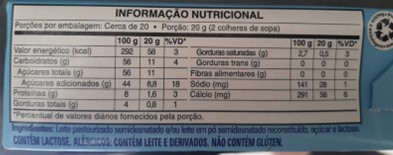
2) Nutrition facts: Improvements in the design, content, and legibility of the nutrition facts panel, in black writing on a white background. Also included the mandatory information of: total and added sugars, number of product servings per package, and all the information on the content of nutrients by 100 g or 100 ml.
3) Nutrition claims: The resolution only has restrictions for nutrition claims of FoPNL, if the package carries a “high in” warning. For example: a product that receives “high in sodium” cannot mention that it has a low content of this nutrient.
The new Brazilian nutrition labeling regulation is an advance for the country, and improves on the pre-existing regulation, which did not have: FoPNL, nutritional facts with specifications for legibility and restriction for nutritional claims. On the other hand, with the approved FoPNL, Brazil falls short of the best practices of the region, which is moving forward warning labels associated with the adoption of PAHO nutrient profile model. This situation shows that Brazil could have done more to safeguard public health, considering the data available at the approval, but was impeded by the unfavorable political context and the food industry interference. This scenario evidenced the need for working towards improving the nutrition food labeling regulation, focused on: improving the nutrient profile model, include information about sweeteners, and the adoption of a package of public policies, based on Latin American positive experiences in Argentina, Mexico and Chile.


